Nuvaxovid
1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic.

Novavax Hopes Its Covid Shot Wins Over Fda Vaccine Holdouts The Boston Globe
Company Novavax should not be given to individuals.

. 1 day agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. 16 fever including 14 severe cases.
Rokotteesta ei myöskään ole haittaa vaikka. On August 19 2022 the Food and Drug Administration FDA authorized the Novavax COVID-19 Vaccine. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
Like the Novavax vaccine side effects were more. Beslutet är temporärt och gäller från. Nu stoppar Folkhälsomyndigheten användningen bland personer som är 30.
88 experienced pain. Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its assessment and approval by the European Medicines Agency.
After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria Astra Zeneca and Covid-19 Vaccine Janssen a further. The Summary of Product Characteristics is a description of a. Information about the COVID-19 vaccine Nuvaxovid approved by the MHRA on 03 February 2022.
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Nuvaxovid contains a version of a protein found on the. The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19.
Nuvaxovid is the first protein-based COVID-19 vaccine granted. Nuvaxovid dispersion for injection. COVID-19 Vaccine recombinant adjuvanted 2.
Name of the medicinal product. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach. Nuvaxovid-rokote sopii lähes kaikille aikuisille.
Data från Australien pekar mot en ökad. The addition of the saponin-based. Det eftersom att data från Australien gett signaler.
Nuvaxovid offers a high level of protection against COVID-19 which is a critical need in the current pandemic. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. This is a multidose vial.
Nuvaxovid vaccine pause for young people justice system spending Västerås shooting young women have more debt than 10 years ago. Qualitative and quantitative composition. Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre.
1 day agoDet proteinbaserade covid-19-vaccinet Nuvaxovid ska inte ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten. Nuvaxovid COVID-19 vaccines are available for use in the United Kingdom as of September 27 2022. Clinical trials showed that the vaccine has around 90 efficacy.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. On December 20 2021 the.
Japan Approves Novavax As 4th Covid Vaccine Amid New Surge Bloomberg
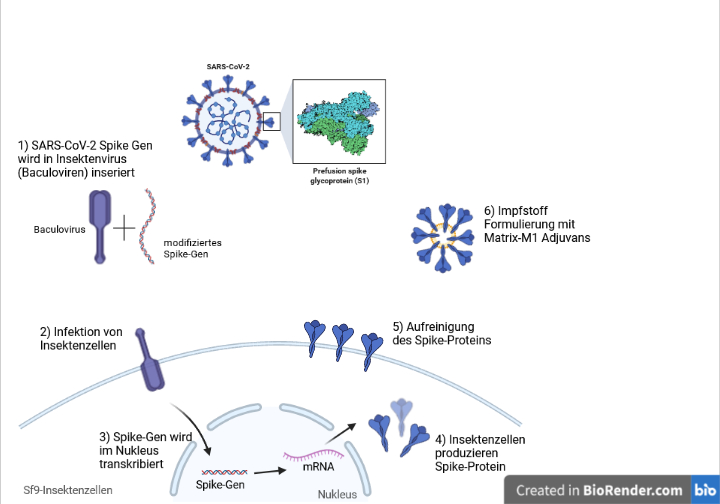
Nuvaxovid The New Subunit Sars Cov 2 Vaccine Mci Innsbruck

Informacie O Covid 19 Nuvaxovid Novavax Vakcine Australian Government Department Of Health And Aged Care

Nuvaxovid Fifth Vaccine Against Covid Authorised In Eu Euractiv Com
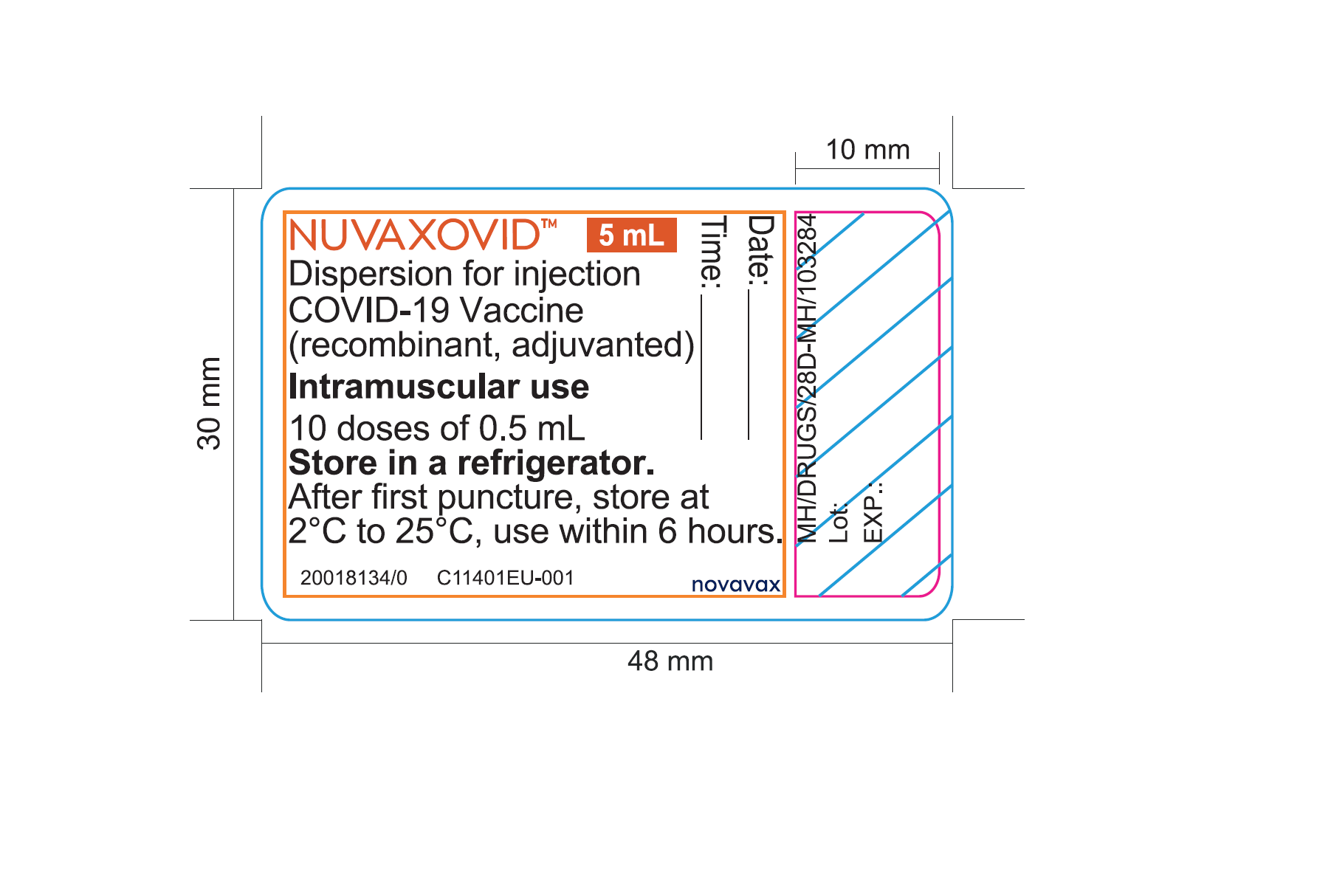
Distribution Of Nuvaxovid With English Only Vial And Carton Labels Canada Ca
Novavax Covid 19 Vaccine Nuvaxovid Provisionally Registered In Australia As A Booster In Individuals Aged 18 And Over Pharmtech Focus

Novavax S Covid 19 Vaccine Candidate Receives Ec Approval For Use As A Booster Pmlive
What Is The Novavax Vaccine And Why Does The World Need Another Type Of Covid 19 Vaccine Gavi The Vaccine Alliance

Informaciya O Vakcine Protiv Covid 19 Nuvaxovid Novavax Australian Government Department Of Health And Aged Care

Novavax S Vaccine Nuvaxovid Vaktsineeri Ee

Could The Novavax Vaccine Help Us Win This Covid War Medpage Today

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre
Who Lists 10th Covid 19 Vaccine For Emergency Use Nuvaxovid Strategic Partnership For Health Security And Emergency Preparedness Sph Portal
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters
Novavax Makes One Million Doses Of Nuvaxovid Available For Use In The United Kingdom Pharmtech Focus
New Protein Based Covid 19 Vaccine Could Help Boost Rates Say Pharmacists Cbc News


